IUMPA-2: intrauterine medroxyprogesterone delivery system in endometrial hyperplasia treatment – first announcement
Andrzej J. Kowalski, Janusz M. Rosiak, Jacek Suzin
 Affiliacja i adres do korespondencji
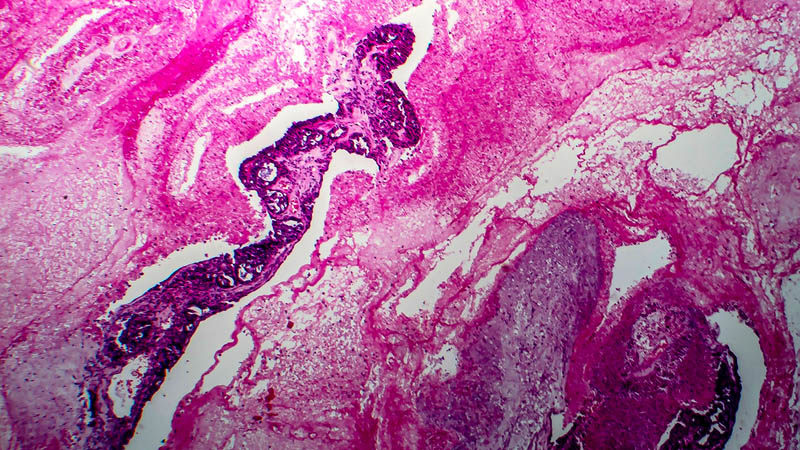
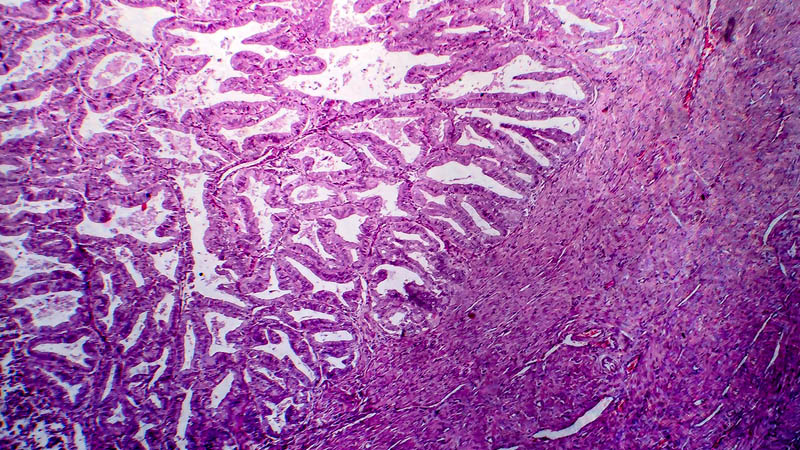
Affiliacja i adres do korespondencjiThe aim of this study was the elaboration of the medroxyprogesterone acetate (MPA) delivery system employed in the treatment of abnormal uterine bleeding, especially in simplex endometrial hyperplasia. Gestagen therapy or surgical procedures were used in such cases till now. Material and methods: IUMPA-2 is the intrauterine device which have 2 grams of medroxyprogesterone acetate. The release of MPA from the carrier has been programmed as the line function within 30 days. The study was carried out in 30 postmenopausal women with histology proven simplex endometrial hyperplasia or ultrasonographical suspicion of endometrial hypertrophy. Results: The posttreatment histology examination performed in all patients revealed: secretion phase or pseudodecidual transformation. Conclusion: IUMPA-2 is the effective topical treatment system for simplex endometrial hyperplasia, offering potential benefits in patients with contraindication for general progesterone application.